Fig. 1: Regional variation of the collagen microstructure of the LADA adventitia. (a) Autofluorescence micrographs from confocal imaging, showing the progressive alignment of the collagen fibers of the adventitia as the LADA descends towards the heart apex. (b) Heatmaps showing the fiber angle probability across the vascular wall as cardiac heart valves in valve replacement applications.
Mechanics-Microstructure Interrelationship for Biological Tissues and its Tissue Engineering (TE) Applications
Background Info: Coronary heart disease (CHD) is the leading cause of death in adults worldwide. CHD is caused by the progressive accumulation of low-density lipoproteins in the coronary endothelium, which triggers an inflammatory response where inflammatory cells and SMCs are recruited to scavenge lipids. This leads to the formation of atherosclerotic plaques, which can reduce the vessel diameter and limit the delivery of oxygenated blood to the myocardium. CHD can be treated using angioplasty or coronary artery bypass graft (CABG) surgery, where CABG is the preferred option for the treatment of severe and multiple occlusions. Approximately 202,000 procedures were performed in 2018 in the United States. However, autologous grafting sources face challenges related to stenosis, anastomotic compliance mismatch, and limited long-term patency. Therefore, there is a critical need for the development of effective non-autologous grafts in CABG surgery. Vascular tissue engineering (VTE) aims to fabricate conduits that can induce vascular wall regeneration. These biomaterial-based conduits should promote complete integration into native tissues, which can potentially lead to biologically active grafts for improved use in CABG surgery.
Research Project: In our lab, one of our overarching goals is to design vascular grafts that can best mimic the mechanical performance and microstructure of native artery tissues for use in CABG surgery. In this project, we plan to systematically study the regional microstructural and biomechanical properties of the left anterior descending artery (LADA) – the coronary artery that is most frequently affected by CHD (see Fig. 1 for example). The data that we will acquire in this project will help us advance the understanding of regional differences in the microstructure of the LADA. This will aid in our efforts to design new, effective tissue-engineered vascular grafts (TEVGs). Similar investigations can also be conducted on other biological tissues, such as cardiac heart valves in valve replacement applications.
Development of Data-Driven/AI (Artificial Intelligence)-Based Computational Modeling Tool for Linking Mechanical Stress and Strain
Background Info: Computational biomechanical modeling of heart valves typically requires the development of a constitutive model with model parameters determined through fitting to mechanical testing data. In contrast, a data-driven (or artificial intelligence (AI)-enabled) computational biomechanics approach directly employs experimental data in conjunction with state-of-the-art machine learning/deep learning algorithm(s) to establish the stress-strain (i.e., mechanical forces vs. deformation) relationship, without the need for conventional constitutive modeling. Such a novel computational modeling paradigm could further be integrated with patient-specific biomechanical simulations to guide diagnosis and surgical planning for valvular heart diseases.
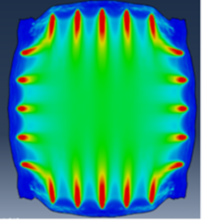
Research Project: In this project, existing mechanical and microstructural data for biological tissues (such as cardiac heart valves, coronary artery vessels, or other biomaterials) will be used. If needed, new or additional data will also be collected to develop a novel data-driven computational modeling approach, as illustrated in Fig. 2. Systematic model calibration and verification will be performed, and the sensitivity of the developed model will be evaluated to ensure its robustness in modeling the target biological systems. The endpoint of this project will be a digital twin of the studied biological system that can produce reliable predictions of its mechanical response. Simulation-enabled information, such as hotspots of mechanical deformations and/or stresses, can serve as guidance for surgeons to make personalized considerations when performing surgical treatment.
Biaxial Testing to Study Cardiovascular Valve Leaflet Biomechanics
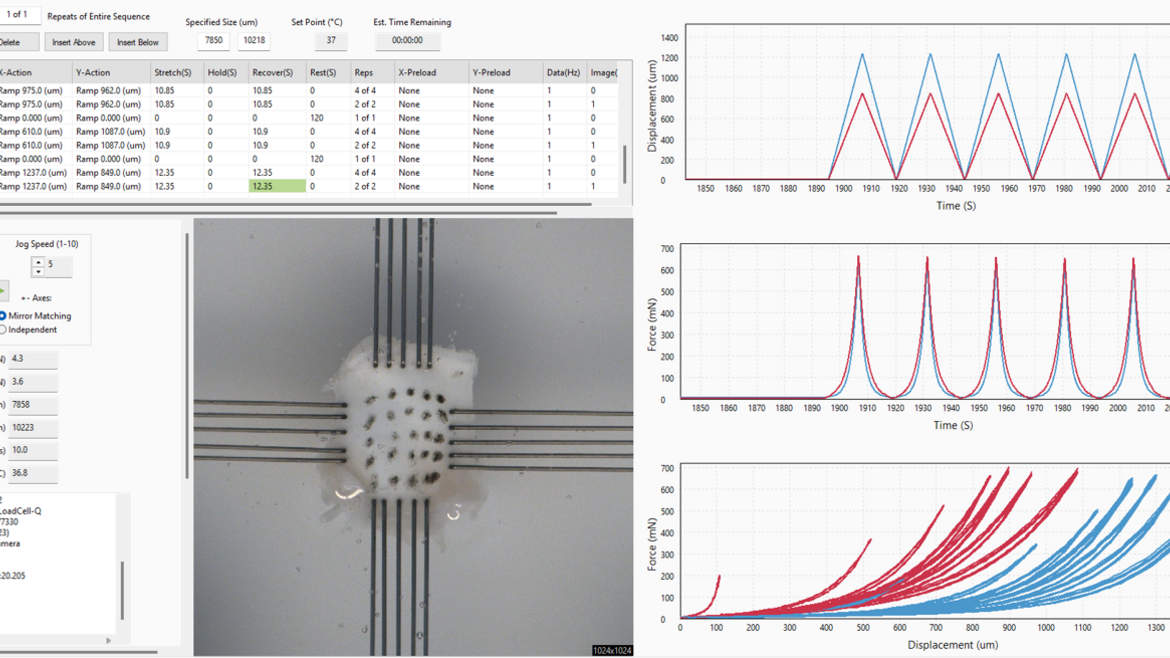
We are committed to advancing the understanding of cardiac tissue biomechanics through mechanical property tests. Our research focuses on biaxial tensile stress testing to evaluate the intricate stress-strain responses of heart valves, particularly the tricuspid and mitral valves. We investigate how these tissues withstand mechanical stress by applying controlled tensile forces, providing critical insights into their structural behavior and functional mechanics. This work is essential in bridging the gap between biological function and material engineering, enabling the development of biomaterials and medical devices that closely mimic the properties of natural heart valves. By integrating experimental data with computational modeling, we aim to design materials and valve replacements that enhance durability and physiological compatibility, ultimately improving treatment outcomes in cardiovascular healthcare.
Opportunities for Students and Collaborators
We welcome inquiries from prospective students, researchers, and collaborators. If you're interested in joining the Biomechanics and Biomaterials Design Lab (BBDL) please fill out this form: Interest Form. If you would like to explore a research collaboration, please contact Dr. Chung-Hao Lee at chunghao.lee@ucr.edu
We look forward to hearing from you!
Research Spotlight
Check out this talk given by one of our talented undergraduate students, Shubhra Singhal, on her research focused on understanding valve function under varying densities of elastin.